Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kato, Hiroshige*; Sato, Mitsuyoshi*; Owada, Hitoshi*; Mihara, Morihiro;
JNC TN8430 2000-008, 53 Pages, 2000/05
Cementitious materials will be used in TRU waste disposal repository. In such cases, it is considered that the migration of alkaline leachates from cementitious materials, so called high pH plume, will cause dissolution of rock and precipitation of secondary minerals. In addition, the high pH plume will move along the flow of groundwater, so it is predicted that rock formation and components of high pH groundwater vary with time and space. However, time and spatial dependence of the variations of secondary minerals and groundwater components has not been clarified. In order to acquire the data of variations of secondary minerals and groundwater components, we carried out the rock alteration experiments with column method. The crushed granodiorite was filled into 4 meters length column (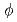 3.7 cm) and artificial cement leachate (pH=13.3; Na=0,1 mol/l, K=0.1 mol/l, Ca=0.002 mol/l) was streamed at flow rates of 0.1 ml/min for 7 months at 80
3.7 cm) and artificial cement leachate (pH=13.3; Na=0,1 mol/l, K=0.1 mol/l, Ca=0.002 mol/l) was streamed at flow rates of 0.1 ml/min for 7 months at 80 C. As the result, secondary minerals confirmed on the rock were calcite and C-S-H at upstream of column and C-S-H at mid-downstream. The pH value of the fluid dominated by Na and K did not be decreased by reaction with the rock. In this study, the data relating to the effect of high pH plume on rock over the long term was acquired.
C. As the result, secondary minerals confirmed on the rock were calcite and C-S-H at upstream of column and C-S-H at mid-downstream. The pH value of the fluid dominated by Na and K did not be decreased by reaction with the rock. In this study, the data relating to the effect of high pH plume on rock over the long term was acquired.
Murakami, Takashi*; Kogure, Toshihiro*; *; Onuki, Toshihiko
American Mineralogist, 83, p.1209 - 1219, 1998/00
no abstracts in English
Isobe, Hiroshi
Kobutsugaku Zasshi, 24(3), p.179 - 186, 1995/07
no abstracts in English
Sasagawa, Tsuyoshi; Kijima, Tatsuya*; Sawaguchi, Takuma; Iida, Yoshihisa
no journal, ,
no abstracts in English
Kijima, Tatsuya*; Sasagawa, Tsuyoshi; Sawaguchi, Takuma; Iida, Yoshihisa
no journal, ,
no abstracts in English
Ichikawa, Nozomi*; Hamamoto, Takafumi*; Sasamoto, Hiroshi; Ichige, Satoru*; Kawakita, Ryohei; Fujisaki, Kiyoshi*
no journal, ,
For model validation of bentonite behavior under high alkaline condition, a batch type reaction experiment and modeling were performed. In the batch type reaction experiment, simulated solutions for the leachate from cement material (Region I solution of pH13 simulated by mixture of both 0.2M NaOH and KOH solutions, Region II solution of pH12.5 simulated by 0.016M CaOH solution) and bentonite (Kunigel V1 and Kunipia F) were reacted under L/S ratio of 50 mL/g and temperature for either 25 or 50 degrees, and for 2 years as the maximum duration. As the results, for example of the experiment for reactions between Region I solution and Kunipia F at 50 degrees, the peak intensity for montmorillonite slightly decreased with time and the new formed secondary mineral suggested as phillipsite occur. Geochemical modeling for this experimental case was performed. Regarding the amount of montmorillonite dissolution, the modeling considering the reactions of both montmorillonite dissolution and ion exchange approximated the experimental result well, however, the addition of the secondary mineral precipitation reaction was less effective. Sensitivity analyses considering the parameter uncertainties of secondary mineral precipitation reaction stimulated that the inconsistency was not dependent on the parameter uncertainties.
solution) and bentonite (Kunigel V1 and Kunipia F) were reacted under L/S ratio of 50 mL/g and temperature for either 25 or 50 degrees, and for 2 years as the maximum duration. As the results, for example of the experiment for reactions between Region I solution and Kunipia F at 50 degrees, the peak intensity for montmorillonite slightly decreased with time and the new formed secondary mineral suggested as phillipsite occur. Geochemical modeling for this experimental case was performed. Regarding the amount of montmorillonite dissolution, the modeling considering the reactions of both montmorillonite dissolution and ion exchange approximated the experimental result well, however, the addition of the secondary mineral precipitation reaction was less effective. Sensitivity analyses considering the parameter uncertainties of secondary mineral precipitation reaction stimulated that the inconsistency was not dependent on the parameter uncertainties.